From Boron Cluster to Two-Dimensional Boron Sheet on Cu(111) Surface: Growth Mechanism and Hole Formation | Scientific Reports

Planar Four‐Coordinate Boron: A Single, Flat Boron Atom as a Ligand for Four Metals - Braunschweig - 2012 - Angewandte Chemie International Edition - Wiley Online Library
307. The number and type of bonds between two boron atoms in B2 molecule :- (1) Two sigma, two pi (2) Two sigma, one pi (3) One Pi (4) One sigma, one pi

Electron-Deficient Heteroacenes that Contain Two Boron Atoms: Near-Infrared Fluorescence Based on a Push–Pull Effect | Organic Chemistry | ChemRxiv | Cambridge Open Engage
When boron atoms bond with atoms of another element, why is it an exception to the octet rule? - Quora

Visualization of the disordered boron atoms forming two kinds of B 12 O... | Download Scientific Diagram

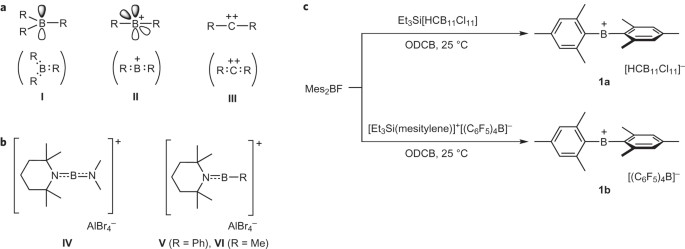
FUKUSHIMA GROUP - Laboratory for Chemistry and Life Science, Institute of Innovative Research, Tokyo Institute of Technology.
Phys. Rev. A 105, 022813 (2022) - Multiple locations of boron atoms in the exohedral and endohedral ${\mathrm{C}}_{60}$ fullerene

Schematic of the two substitution configurations: boron atom (in grey)... | Download Scientific Diagram

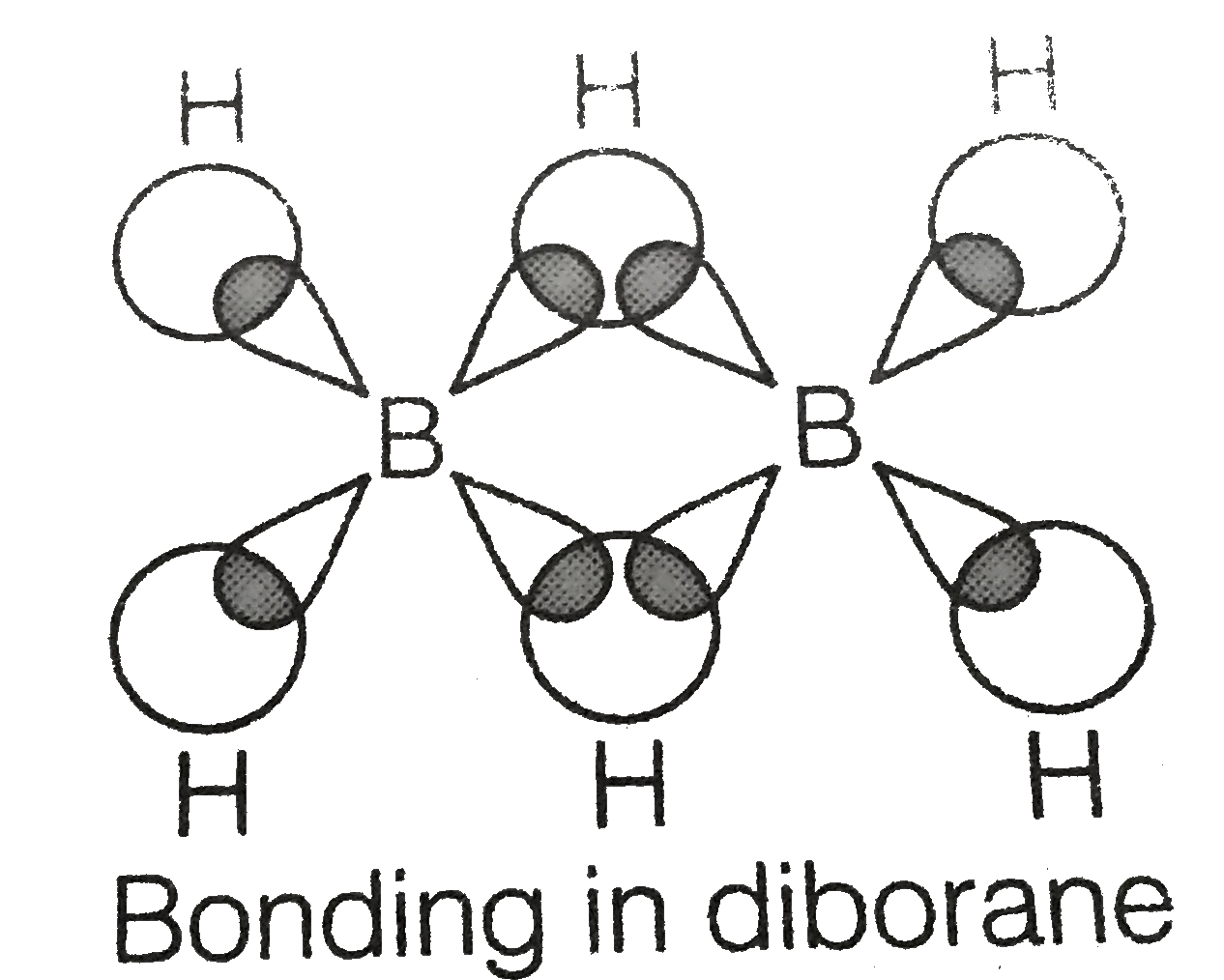
Octahydriodo diborane (B2H8) and its protonated cations containing five-, six-, and seven-coordinate boron atoms | PNAS